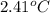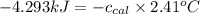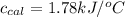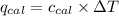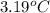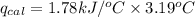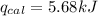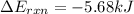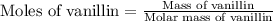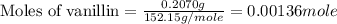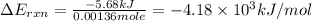The combustion of 0.1625 g benzoic acid increases the temperature of a bomb calorimeter by 2.41°c. calculate the heat capacity of this calorimeter. (the energy released by combustion of benzoic acid is 26.42 kj/g.)
kj/°c
a 0.2070 g sample of vanillin (c8h8o3) is then burned in the same calorimeter, and the temperature increases by 3.19°c. what is the energy of combustion per gram of vanillin?
kj/g
what is the energy of combustion per mole of vanillin?
kj/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mimithurmond03
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
The combustion of 0.1625 g benzoic acid increases the temperature of a bomb calorimeter by 2.41°c. c...
Questions in other subjects:

English, 21.11.2019 00:31




Mathematics, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31




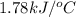
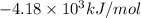
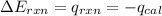
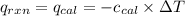
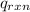 = heat released by the reaction = -4.293 kJ
= heat released by the reaction = -4.293 kJ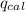 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter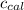 = specific heat of calorimeter = ?
= specific heat of calorimeter = ? = change in temperature =
= change in temperature =