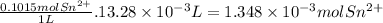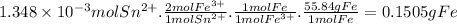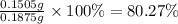Chemistry, 07.11.2019 03:31 tanaemichel
The metal content of iron in ores can be determined by a redox procedure in which the sample is first oxidized with br2 to convert all the iron to fe3+ and then titrated with sn2+ to reduce the fe3+ to fe2+.
the balanced equation is: 2fe3+(aq) + sn2+(aq) -> 2fe2+(aq) + sn4+(aq)
what is the mass percentage fe in a 0.1875g sample if 13.28 ml of 0.1015 m sn2+ solution is needed to titrate the fe3+?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, 1020lakyn
Subduction zones occur on earth where dense oceanic crust dives under more buoyant continental crust. these boundaries are characterized by a deep ocean trench next to a high continental mountain range, large numbers of earthquakes and volcanoes. all of this is further evidence for the a) big bang theory b) origin of the species eliminate c theory of plate tectonics d theory of natural selection 4 sedimentary rocks found high in the himalayen mountain
Answers: 1



Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
The metal content of iron in ores can be determined by a redox procedure in which the sample is firs...
Questions in other subjects:



Mathematics, 22.05.2020 22:57

Mathematics, 22.05.2020 22:57



Mathematics, 22.05.2020 22:57