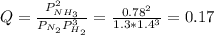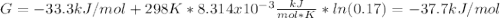In the haber process, ammonia is synthesized from nitrogen and hydrogen: n2(g)+3h2(g)→2nh3(g) δg∘ at 298k for this reaction is −33.3kj/mol. the value of δg at 298k for a reaction mixture that consists of 1.3atmn2, 1.4atmh2, and 0.78atmnh3 is kj/mol. in the haber process, ammonia is synthesized from nitrogen and hydrogen: at for this reaction is . the value of at for a reaction mixture that consists of , , and is . −4.42×103 −76.6 −37.7 −5.7 −2.13 × 103

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, montoyaricardo3550
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
In the haber process, ammonia is synthesized from nitrogen and hydrogen: n2(g)+3h2(g)→2nh3(g) δg∘ a...
Questions in other subjects:

Mathematics, 16.02.2021 18:50


Mathematics, 16.02.2021 18:50


Mathematics, 16.02.2021 18:50


Mathematics, 16.02.2021 18:50

Computers and Technology, 16.02.2021 18:50

Mathematics, 16.02.2021 18:50

Mathematics, 16.02.2021 18:50

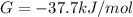
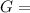 Δ
Δ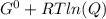
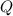 is computed via the law of mass action:
is computed via the law of mass action: