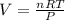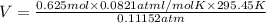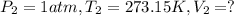Chemistry, 07.11.2019 01:31 KariSupreme
Calculate the experimental molar volume (l/mol) for an ideal gas at stp using the information that follows. a 15.0 mg piece of solid magnesium was reacted completely with hydrochloric acid in a reaction flask with a volume of 135 ml. the temperature of the reaction was 22.3 °c and the pressure of the gas produced by the reaction was 11.3 kpa. calculate the volume of hydrogen gas that would have formed in this reaction had it been conducted under standard temperature and pressure conditions (use the combined gas law). use the volume you have just determined, along with the number of moles of hydrogen gas that would have formed from 15.0 mg of magnesium reactant, to calculate the molar volume of this gas at stp

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, thickness7699
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1
You know the right answer?
Calculate the experimental molar volume (l/mol) for an ideal gas at stp using the information that f...
Questions in other subjects:

English, 17.10.2019 15:30

Biology, 17.10.2019 15:30


Advanced Placement (AP), 17.10.2019 15:30

Mathematics, 17.10.2019 15:30


History, 17.10.2019 15:30


Mathematics, 17.10.2019 15:30

Mathematics, 17.10.2019 15:30

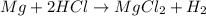
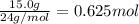
 of hydrogen gas.
of hydrogen gas. (ideal gas equation)
(ideal gas equation)