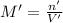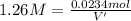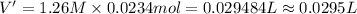Let us assume that cr(oh)3(s) is completely insoluble, which signifies that the precipitation reaction with naoh(aq) (presented in the transition) would go to completion.
cr3+(aq)+3naoh(aq) → cr(oh)3(s)+3na+(aq)
if you had a 0.600 l solution containing 0.0130 m of cr3+(aq), and you wished to add enough 1.26 m naoh(aq) toprecipitate all of the metal, what is the minimum amount of the naoh(aq) solution you would need to add? assume that the naoh(aq) solution is the only source of oh−(aq) for the precipitation.
express the volume to three significant figures and include the appropriate units.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 15:30, vivianfling
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Let us assume that cr(oh)3(s) is completely insoluble, which signifies that the precipitation reacti...
Questions in other subjects:

History, 14.12.2019 09:31


Mathematics, 14.12.2019 09:31

Mathematics, 14.12.2019 09:31

English, 14.12.2019 09:31


English, 14.12.2019 09:31

Mathematics, 14.12.2019 09:31


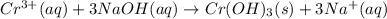
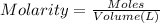
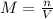
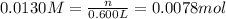
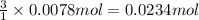 of NaOH
of NaOH