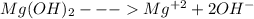Chemistry, 07.11.2019 01:31 shimmerandshine1
The ksp of magnesium hydroxide, mg(oh)2, is 1.2 x 10-11, so setting 4s3 = ksp, results in s = 1.4 x 10-4. this leads to an [oh-] = 2.8 x 10-4 (i. e., a poh=3.55), thus a ph of 10.45. given the aforementioned information and resulting calculations, comment on the solubility of mg(oh)2 at ph values: 1) less than 10.45 and, 2) greater than 10.45

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2


Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1
You know the right answer?
The ksp of magnesium hydroxide, mg(oh)2, is 1.2 x 10-11, so setting 4s3 = ksp, results in s = 1.4 x...
Questions in other subjects:

Mathematics, 05.02.2021 20:00






Physics, 05.02.2021 20:00

History, 05.02.2021 20:00