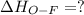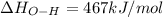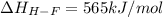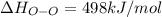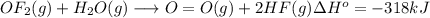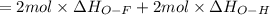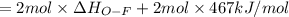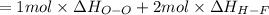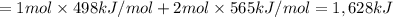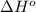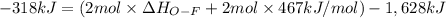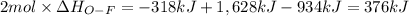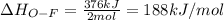Chemistry, 07.11.2019 01:31 jenlicavoli
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy of the o–f bond using the standard enthalpy of reaction and the bond energy data provided. just enter a number (no units). of2(g) + h2o(g) \longrightarrow⟶ o=o(g) + 2hf(g) \deltaδh° = –318 kj bond: o–h o=o h–f bond energy (kj/mol): 467 498 565

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3



Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1
You know the right answer?
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy...
Questions in other subjects:




SAT, 18.11.2021 20:10

Business, 18.11.2021 20:30




Biology, 18.11.2021 20:50

Mathematics, 18.11.2021 21:00