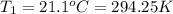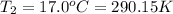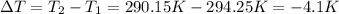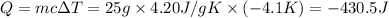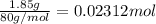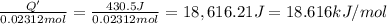Instant cold packs used to ice athletic injuries on the field contain ammonium nitrate and water separated by a thin plastic divider. when the divider is broken, the ammonium nitrate dissolves according to the endothermic reaction: nh4no3 (s) --> nh4+ (aq) + no3- (aq) 1.85g ammonium nitrate is added to water in a calorimeter. the total solution (water and ammonium nitrate) is 25.0g. the heat capacity of the calorimeter is ccal=45.0 j/k. the initial temperature of the solution is 21.1c. the final temperature of the solution is 17.0c. assume cs of the solution is 4.20 j/(g k) what is δhrxn per mol of the reaction?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1


Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Instant cold packs used to ice athletic injuries on the field contain ammonium nitrate and water sep...
Questions in other subjects:



Mathematics, 12.08.2020 05:01





Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01