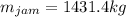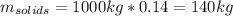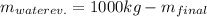Chemistry, 06.11.2019 05:31 student0724
Production of jam from crushed fruit in two stages. in a process producing jam (c1), crushed fruit containing 14 wt % soluble solids is mixed in a mixer with sugar (1.22 kg sugar/1.00 kg crushed fruit) and pectin (0.0025 kg pectin/1.00 kggensymb crushed fruit). the resultant mixture is then evaporated in a kettle to produce a jam containing 67 wt% soluble solids. for a feed of 1000 kg crushed fruit, calculate the kg mixture from the mixer, kg water evaporated, and kg jam produced

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, kitykay2776
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 10:30, lavorisjonesjr1
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3

Chemistry, 23.06.2019 11:00, randyg0531
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
Production of jam from crushed fruit in two stages. in a process producing jam (c1), crushed fruit c...
Questions in other subjects:

Biology, 30.09.2019 20:30



Mathematics, 30.09.2019 20:30


Mathematics, 30.09.2019 20:30

Mathematics, 30.09.2019 20:30