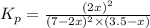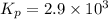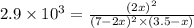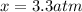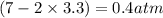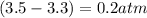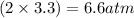Chemistry, 06.11.2019 04:31 PlsHelpMeh3401
Calculate the pressures of no, cl2, and nocl in an equilibrium mixture produced by the reaction of a starting mixture with 7.0 atm no and 3.5 atm cl2. (hint: kp is relatively large; assume the reaction goes to completion then comes back to equilibrium.)
2 no(g) + cl2(g) --> 2 nocl(g)kp = 2.9 ✕ 103 at 149°c

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
Calculate the pressures of no, cl2, and nocl in an equilibrium mixture produced by the reaction of a...
Questions in other subjects:


History, 03.02.2020 14:44

Biology, 03.02.2020 14:44

Biology, 03.02.2020 14:44


English, 03.02.2020 14:44

Mathematics, 03.02.2020 14:44


English, 03.02.2020 14:44

History, 03.02.2020 14:44

 ,
, 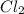 , and
, and 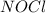 in an equilibrium mixture are 0.4 atm , 0.2 atm and 6.6 atm respectively.
in an equilibrium mixture are 0.4 atm , 0.2 atm and 6.6 atm respectively.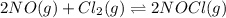
![K_p=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0361/4899/09f8c.png)