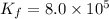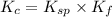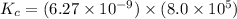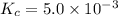Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, cynthiagutierrez65
Where can i find naap lab answers sheet/key?
Answers: 1

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

You know the right answer?
Calculate the value of the equilibrium constant, kc, for the reaction cubr(s) + br−(aq) ↽⇀ cubr−2(aq...
Questions in other subjects:

Mathematics, 19.07.2019 02:00

Mathematics, 19.07.2019 02:00





Biology, 19.07.2019 02:00



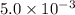
![CuBr(s)+Br^-(aq)\rightleftharpoons [CuBr_2]^-(aq)](/tpl/images/0361/4240/23bce.png)
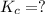
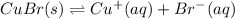
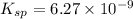
![Cu^+(aq)+2Br^-(aq)\rightleftharpoons [CuBr_2]^-(aq)](/tpl/images/0361/4240/ae2b9.png)