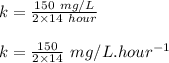Engineers do a treatment study on an industrial waste containing chemical x. in a batch reactor, the concentration of chemical x drops from 150 mg/l to 75 mg/l in 14 hours.
(a) what is the half-life?
(b) what is the decay constant if the reaction is first order?
(c) what is the decay constant if the reaction is zero order?
(d) how would you determine whether the reaction is zero or first order? if you had a set of concentration

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 17:00, pinkypie123457
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2
You know the right answer?
Engineers do a treatment study on an industrial waste containing chemical x. in a batch reactor, the...
Questions in other subjects:








Arts, 10.11.2020 16:20

English, 10.11.2020 16:20

Biology, 10.11.2020 16:20

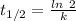
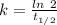
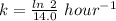
![t_{1/2}=\frac{[A]_0}{2k}](/tpl/images/0361/2925/15a8b.png)
![[A]_0](/tpl/images/0361/2925/7075c.png) is the initial concentration = 150 mg/L
is the initial concentration = 150 mg/L