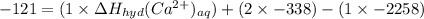Chemistry, 06.11.2019 02:31 wirchakethan23
The heat of solution of calcium chloride is -121 kj/mol. given that the lattice energy of calcium chloride is -2258 kj/mol and the heat of hydration of a chloride ion is -338 kj/mol calculate the heat of hydration of a calcium ion.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1
You know the right answer?
The heat of solution of calcium chloride is -121 kj/mol. given that the lattice energy of calcium ch...
Questions in other subjects:

Physics, 10.06.2020 10:57

Physics, 10.06.2020 10:57


Mathematics, 10.06.2020 10:57





Mathematics, 10.06.2020 10:57

Computers and Technology, 10.06.2020 10:57

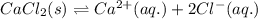
![\Delta H_{sol}=[1mol\times \Delta H_{hyd}(Ca^{2+})_{aq.}]+[2mol\times \Delta H_{hyd}(Cl^{-})_{aq.}]-[1mol\times U(CaCl_{2})_{s}]](/tpl/images/0361/2975/af20b.png)
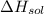 is heat of solution,
is heat of solution, 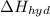 is heat of hydration and U represents lattice energy.
is heat of hydration and U represents lattice energy.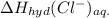 = -338 kJ/mol and
= -338 kJ/mol and 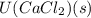 = -2258 kJ/mol
= -2258 kJ/mol