
Chemistry, 06.11.2019 02:31 kimbely7704
The molar heat capacity of ethane is represented in the temperature range 298 k to 400 k by the empirical expression cp, m in j k1 mol 14.73 + (0.1272 t in k). the corresponding expressions for c(e) and h2(g) are given in the back of the atkins textbook. calculate the standard enthalpy of formation of ethane at 373 k from its value at 298 k, in kj mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:00, crysderria
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1


Chemistry, 23.06.2019 02:40, towelmearowel
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
The molar heat capacity of ethane is represented in the temperature range 298 k to 400 k by the empi...
Questions in other subjects:

Chemistry, 06.10.2019 16:30



Computers and Technology, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30

English, 06.10.2019 16:30

English, 06.10.2019 16:30


History, 06.10.2019 16:30

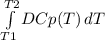
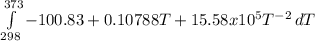 = -3796.48 J/mol = -3.80 kJ/mol (solved by a graphic calculator)
= -3796.48 J/mol = -3.80 kJ/mol (solved by a graphic calculator)

