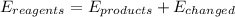Which of the statements are true? select all that apply. a) the entropy at the start of a reaction is always greater than the entropy of the products. b) the amount of usable energy resulting from a reaction is always less than the total energy available at the start of the reaction. c) the entropy of the products of a reaction is always greater than the entropy at the start of the reaction. d) the energy for a reaction equals the sum of the energy in the product plus energy released as heat and disorder.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Which of the statements are true? select all that apply. a) the entropy at the start of a reaction...
Questions in other subjects:





Biology, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01