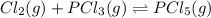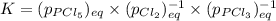Which equilibrium constant expression(s) are for the following reaction. (x stands for the mole fraction of the indicated substance in its phase, which we have so far assumed equal to unity (one) for essentially pure phases.)
cl2(g) + pcl3(g)↔pcl5(g))
choose from the list below and enter the letters alphabetical order. (e. g. ah)
(a) (pcl2)eq
(b) (ppcl3)eq
(c) (ppcl5)eq
(d) (pcl2)eq-1
(e) (ppcl3)eq-1
(f) (ppcl5)eq-1
(g) (pcl2)eq2
(h) (ppcl3)eq3
(i) (ppcl5)eq5
(j) (pcl2)eq-2
(k) (ppcl3)eq-3
(l) (ppcl5)eq-5

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, stephaniero6
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3



Chemistry, 23.06.2019 00:30, zaniathomasel
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Which equilibrium constant expression(s) are for the following reaction. (x stands for the mole frac...
Questions in other subjects:

Biology, 29.01.2020 13:42

Mathematics, 29.01.2020 13:42

Mathematics, 29.01.2020 13:42

Social Studies, 29.01.2020 13:42


Mathematics, 29.01.2020 13:42

Mathematics, 29.01.2020 13:42

Mathematics, 29.01.2020 13:42

English, 29.01.2020 13:42

 .
.