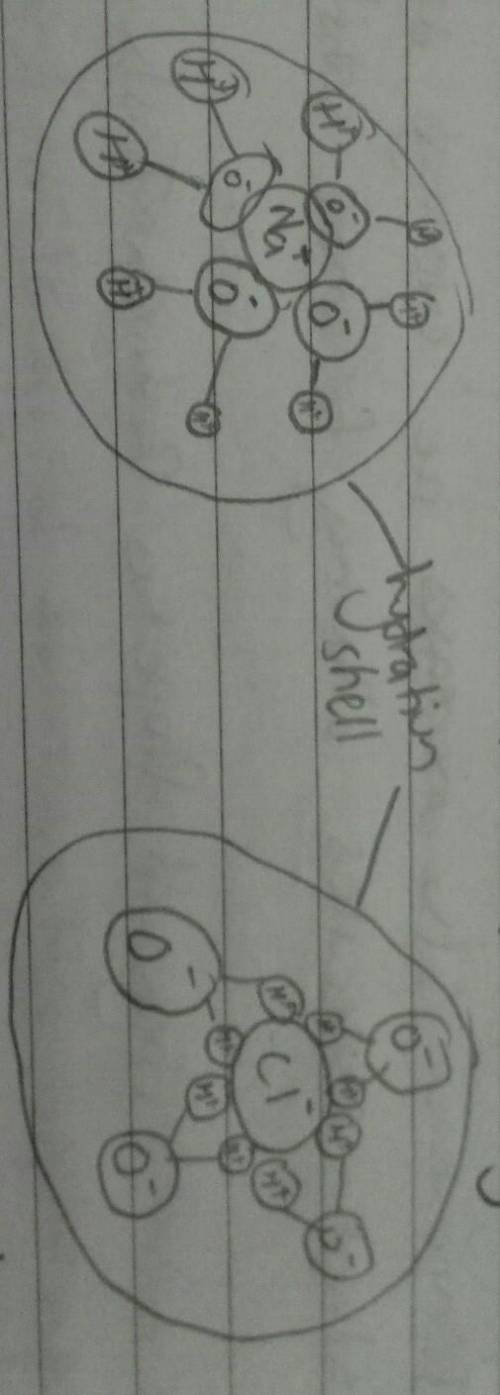
Chemistry, 05.11.2019 09:31 pearljammarow6ujs
Part a: explain why and how polar covalent bonds found in water molecules are responsible for waters ability to dissolve many substances, particularly ionic compounds such as salt.
part b: identify the solvent and the solute in the solution that has been created in part a from dissolving salt in water.
for any : )

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:20, banna01man
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2


Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Part a: explain why and how polar covalent bonds found in water molecules are responsible for water...
Questions in other subjects:

Social Studies, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01