
Chemistry, 05.11.2019 04:31 angelapegues20097
A22.4 l high pressure reaction vessel is charged with 0.5020 mol of iron powder and 1.39 atm of oxygen gas at standard temperature. on heating, the iron and oxygen react according to the balanced reaction below. 4fe(s) + 3o2(g) → 2fe2o3(s) after the reaction vessel is cooled, and assuming the reaction goes to completion, what pressure of oxygen remains?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

You know the right answer?
A22.4 l high pressure reaction vessel is charged with 0.5020 mol of iron powder and 1.39 atm of oxyg...
Questions in other subjects:



Mathematics, 29.10.2020 23:30

Biology, 29.10.2020 23:30

Mathematics, 29.10.2020 23:30

Mathematics, 29.10.2020 23:30


Chemistry, 29.10.2020 23:30


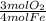 = 0.3765 mol O₂
= 0.3765 mol O₂


