
Chemistry, 05.11.2019 03:31 paytonpaige22
Aluminum reacts with excess aqueous hydrochloric acid to produce hydrogen. (a) write a balanced chemical equation for the reaction. (hint: water-soluble alcl3 is the stable chloride of aluminum.) (b) calculate the mass of pure aluminum that will furnish 10.0 l hydrogen at a pressure of 0.750 atm and a temperature of 30.0°c.

Answers: 1


Other questions on the subject: Chemistry




You know the right answer?
Aluminum reacts with excess aqueous hydrochloric acid to produce hydrogen. (a) write a balanced chem...
Questions in other subjects:

English, 13.09.2021 15:10

Mathematics, 13.09.2021 15:10


Business, 13.09.2021 15:10

Mathematics, 13.09.2021 15:10

Mathematics, 13.09.2021 15:10




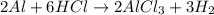
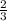 mole of aluminum is consumed.
mole of aluminum is consumed.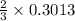 mole of aluminum is consumed.
mole of aluminum is consumed.

