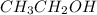Chemistry, 05.11.2019 02:31 devbar3416
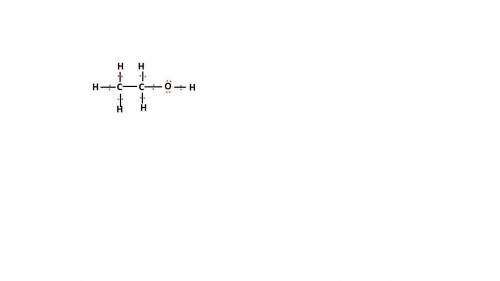
Write the lewis structure for ethanol (ch3ch2oh), the alcohol found in alcoholic beverages, then answer the following questions: 1. how many valence electrons does this alcohol have? 2. how many bonded electrons does this alcohol have? 3. how many lone pairs of electrons does this alcohol have? 4. how many single bonds does this alcohol have?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2


Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Write the lewis structure for ethanol (ch3ch2oh), the alcohol found in alcoholic beverages, then ans...
Questions in other subjects:

Mathematics, 24.12.2019 07:31

Mathematics, 24.12.2019 07:31


Mathematics, 24.12.2019 07:31

Health, 24.12.2019 07:31



Mathematics, 24.12.2019 07:31

World Languages, 24.12.2019 07:31

Biology, 24.12.2019 07:31