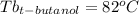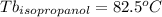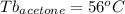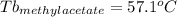Chemistry, 05.11.2019 02:31 Tweektweak
Astudent is given an unknown, clear, colorless liquid at room temperature. the student measures the density, melting point, and boiling point of the liquid. what would the student conclude if he or she found out that the unknown had a density of .79 g/cm3 and a boiling point is 82.05°c? a the substance is t-butanol b the substance is isopropanol c the substance is acetone d the substance is methyl acetate

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1



Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Astudent is given an unknown, clear, colorless liquid at room temperature. the student measures the...
Questions in other subjects:


Chemistry, 02.05.2021 04:00


French, 02.05.2021 04:00

Mathematics, 02.05.2021 04:00



Mathematics, 02.05.2021 04:00


Physics, 02.05.2021 04:00