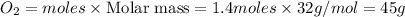Question 51 pts aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent, desiccant, or catalyst for organic reactions. a mixture of 82.49 g of aluminum and 117.65 g of oxygen is allowed to react. what is the mass of the excess reactant present in the vessel when the reaction is complete? report your answer to the appropriate number of significant figures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, rileyallen4186pd5tgy
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1


Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Question 51 pts aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorb...
Questions in other subjects:

Mathematics, 05.05.2020 09:30


Mathematics, 05.05.2020 09:30

Chemistry, 05.05.2020 09:30

Business, 05.05.2020 09:30



Mathematics, 05.05.2020 09:30


Mathematics, 05.05.2020 09:30

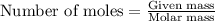
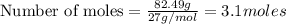
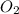
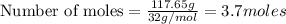
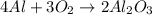
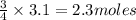 of
of