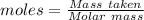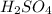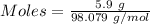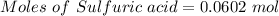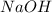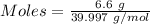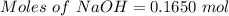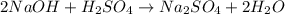Chemistry, 05.11.2019 00:31 safiyabrowne7286
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sulfate (na2so4) and liquid water (h2o). what is the theoretical yield of water formed from the reaction of 5.9 g of sulfuric acid and 6.6 g of sodium hydroxide?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3


Chemistry, 23.06.2019 05:30, xarianna2007
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sul...
Questions in other subjects:

Social Studies, 20.07.2021 19:40





Mathematics, 20.07.2021 19:40



Physics, 20.07.2021 19:40