Chemistry, 04.11.2019 23:31 pinkycupcakes3oxbqhx
Apowder contains feso4⋅7h2o (molar mass=278.01 g/mol), among other components. a 2.955 g sample of the powder was dissolved in hno3 and heated to convert all iron to fe3+. the addition of nh3 precipitated fe2o3⋅xh2o, which was subsequently ignited to produce 0.201 g fe2o3. what was the mass of feso4⋅7h2o in the 2.955 g sample?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 03:00, dad46
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
Apowder contains feso4⋅7h2o (molar mass=278.01 g/mol), among other components. a 2.955 g sample of t...
Questions in other subjects:


History, 13.06.2020 21:57

Mathematics, 13.06.2020 21:57







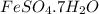 =
= 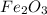
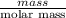
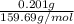
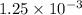 mol
mol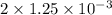 mol
mol