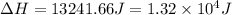Chemistry, 04.11.2019 23:31 mentatmenot
Calculate the enthalpy change (in joules) involved in converting 5.00 grams of water at 14.0 °c to steam at 115 °c under a constant pressure of 1 atm. the specific heats of ice, liquid water, and steam are, respectively, 2.03, 4.18, 1.84 j/g-k and for water δhfusion = 6.01 kj/mole and δhvap = 40.67 kj/mole
a. 1.32x10^4 j
b. 2.05 x 10^5 j
c. 195x 10^3 j
d. 1.94 x 10^3 j

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
You know the right answer?
Calculate the enthalpy change (in joules) involved in converting 5.00 grams of water at 14.0 °c to s...
Questions in other subjects:



Mathematics, 10.12.2020 17:00

Mathematics, 10.12.2020 17:00

Mathematics, 10.12.2020 17:00


History, 10.12.2020 17:00


Physics, 10.12.2020 17:00

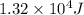
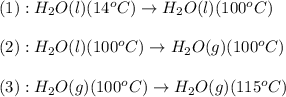
![\Delta H=[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0359/4713/cc64b.png)
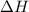 = enthalpy change or heat required = ?
= enthalpy change or heat required = ?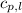 = specific heat of liquid water =
= specific heat of liquid water = 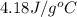
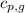 = specific heat of liquid water =
= specific heat of liquid water = 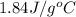
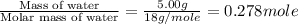
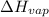 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[5.00g\times 4.18J/g^oC\times (100-14)^oC]+0.278mole\times 40670J/mole+[5.00g\times 1.84J/g^oC\times (115-100)^oC]](/tpl/images/0359/4713/26444.png)