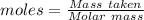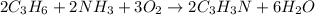Chemistry, 04.11.2019 21:31 justhereforanswers13
Acrylonitrile c3h3n is the starting material for the production of a type of widely used plastic called acrylics. acrylonitrile can be prepared from propylene, c3h6 by reaction with ammonia and oxygen. find the percent yield of acrylonitrile if the reaction yield is 91g when 84 g of propylene and 34 g of ammonia are reacted

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 01:00, deidaralove90
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3
You know the right answer?
Acrylonitrile c3h3n is the starting material for the production of a type of widely used plastic cal...
Questions in other subjects:


Mathematics, 14.04.2021 03:50




Arts, 14.04.2021 03:50


Mathematics, 14.04.2021 03:50


Mathematics, 14.04.2021 03:50