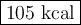Chemistry, 04.11.2019 07:31 elizabethwaller8104
How much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen?
n2(g)+o2(g)→2no(g), δh = + 43 kcal/molhow much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen?
n2(g)+o2(g)→2no(g), δh = + 43 kcal/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, Islandgirl67
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b. zinc7.14,c. copper 8.92,d. lead 11.34
Answers: 2


Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
How much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen...
Questions in other subjects: