
Chemistry, 02.11.2019 07:31 morganhines181
Solution of the schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 1 the quantum number l can have values from to . the total number of orbitals possible at the n = 1 energy level is . if the value of l = 1 the quantum number ml can have values from to . the total number of orbitals possible at the l = 1 sublevel is .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1


Chemistry, 23.06.2019 03:30, LlayahHarbin
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
Solution of the schrödinger wave equation for the hydrogen atom results in a set of functions (orbit...
Questions in other subjects:


Mathematics, 25.01.2021 23:40


History, 25.01.2021 23:40

History, 25.01.2021 23:40



Computers and Technology, 25.01.2021 23:40


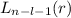 , being n and l the quantum numbers. As is known, in the Laguerre polynomials the subindex must be greater than or equal to 0, which implies
, being n and l the quantum numbers. As is known, in the Laguerre polynomials the subindex must be greater than or equal to 0, which implies 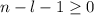 . So if n=1, the only possible value of l is l=0.
. So if n=1, the only possible value of l is l=0. ![Y_{l,m_l}(\theta,\phi)[\tex] as solution. One of the properties of spherical harmonics is that the number [tex]m_l[\tex] can only take the values [tex]m_l=-l,-l+1,-l+2,...,l-1,l[\tex]. So if l=0, as above, [tex]m_l[\tex] can only take the value [tex]m_l=0,[\tex] thus giving only 1 orbital. But if l=1, [tex]m_l](/tpl/images/0356/6953/a304c.png) can be
can be 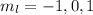 . Therefore the sublevel l=| has 3 possible orbitals.
. Therefore the sublevel l=| has 3 possible orbitals.

