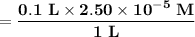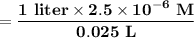Chemistry, 02.11.2019 05:31 Ezekielcassese
Astudent in lab prepared a cereal sample solution for spectroscopic analysis as described in the lab manual, and found the concentration of fe3+ in the sample to be 2.50 x 10−5 m. if her sample solution was prepared by diluting 25 ml of the original solution to a total volume of 100 ml, what is the concentration of the original solution?

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Astudent in lab prepared a cereal sample solution for spectroscopic analysis as described in the lab...
Questions in other subjects:

Mathematics, 14.11.2019 21:31








Chemistry, 14.11.2019 21:31

Mathematics, 14.11.2019 21:31