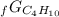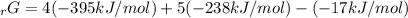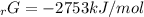Chemistry, 02.11.2019 05:31 WhiteMex69
Given the values of δgfo given below in kj/mol, calculate the value of δgo in kj for the combustion of 1 mole of butane to form carbon dioxide and liquid water. δgfo (c4h10(g)) = -17 δgfo (co2(g)) = -395 δgfo (h2o(l)) = -238

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
You know the right answer?
Given the values of δgfo given below in kj/mol, calculate the value of δgo in kj for the combustion...
Questions in other subjects:

Mathematics, 04.05.2021 02:10


Mathematics, 04.05.2021 02:10

Mathematics, 04.05.2021 02:10

Mathematics, 04.05.2021 02:10


Mathematics, 04.05.2021 02:10


Mathematics, 04.05.2021 02:10

Mathematics, 04.05.2021 02:10


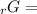 4Δ
4Δ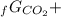 5Δ
5Δ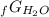 -Δ
-Δ