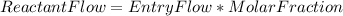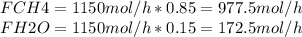Chemistry, 02.11.2019 04:31 Svetakotok
Steam reforming of natural gas is the most common method of producing commercial hydrogen. a stream with a flow rate of 1150 mol/h containing 85.0 mol% ch4 and 15.0 mol% of water is combined with additional water steam and fed to a steam reforming reactor to produce hydrogen. the stream coming out is in chemical equilibrium. the fractional conversion for both the water and methane are 0.600. balance the chemical equation and calculate how much additional water steam is fed to the steam reforming reactor and the flow rate of the outlet hydrogen. ch4 + h2o < --> co + 3h2 (balanced)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

You know the right answer?
Steam reforming of natural gas is the most common method of producing commercial hydrogen. a stream...
Questions in other subjects:

Mathematics, 06.01.2021 01:00

Mathematics, 06.01.2021 01:00


Mathematics, 06.01.2021 01:00

Mathematics, 06.01.2021 01:00

Mathematics, 06.01.2021 01:00

History, 06.01.2021 01:00


Social Studies, 06.01.2021 01:00