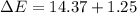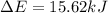Chemistry, 02.11.2019 03:31 Jsanders2276
In a piston, the addition of 14.37 kj of heat to a 100. g sample of a liquid at a constant temperature of 35.2 °c caused the liquid to vaporize (change to a gas). the vaporized gas expanded against an external pressure of 1.07 atm and a volume change of 11.49 l was observed. (recall: 1 l• atm = 101.3 j} what was the change in the internal energy of the system, (ae in kj)? (enter your answer with two decimals places and no units.)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1
You know the right answer?
In a piston, the addition of 14.37 kj of heat to a 100. g sample of a liquid at a constant temperatu...
Questions in other subjects:

History, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

English, 18.09.2020 04:01

Social Studies, 18.09.2020 04:01

Social Studies, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

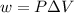
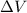 = change in volume = 11.49 L
= change in volume = 11.49 L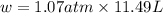
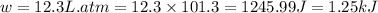 (as per conversion)
(as per conversion)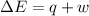
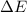 = internal energy of the system
= internal energy of the system