
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)2kcl(s) + 3o2(g) the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 749 mm hg. if the wet o2 gas formed occupies a volume of 5.76 l, the number of moles of kclo3 reacted was mol. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo...
Questions in other subjects:



Mathematics, 27.04.2021 18:50

Mathematics, 27.04.2021 18:50


English, 27.04.2021 18:50

Mathematics, 27.04.2021 18:50

Mathematics, 27.04.2021 18:50


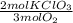 = 0,15 mol of KClO₃
= 0,15 mol of KClO₃


