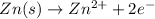Chemistry, 22.01.2020 03:31 alyssasnyderrr
Given the balanced ionic equation:
zn(s) + cu2+(aq) → zn2+(aq) + cu(s)
which equation represents the oxidation half reaction?
(1) zn(s) + 2e– → zn2+(aq)
(2) zn(s) → zn2+(aq) + 2e–
(3) cu2+(aq) → cu(s) + 2e–
(4) cu2+(aq) + 2e– → cu(s)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1



Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Given the balanced ionic equation:
zn(s) + cu2+(aq) → zn2+(aq) + cu(s)
which equation r...
zn(s) + cu2+(aq) → zn2+(aq) + cu(s)
which equation r...
Questions in other subjects:

Mathematics, 04.03.2021 09:50


Mathematics, 04.03.2021 09:50

English, 04.03.2021 09:50


Advanced Placement (AP), 04.03.2021 14:00