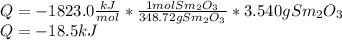A3.540-g sample of an unknown metal m is burned in the presence of excess oxygen, producing the oxide m2o3(s) and liberating 18.56 kj of heat at constant pressure. what is the identity of the metal? 4m(s) + 3o2(g) → 2m2o3(s) substanceδh°f (kj/mol) yb2o3(s)–1814.6 tb2o3(s)–1865.2 sm2o3(s)–1823.0 sc2o3(s)–1908.8 y2o3(s)–1905.3 a) sm b) tb c) y d) sc e) yb

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, ashtonviceoxd21i
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b. colder climates near the equator c. large waves on the cost of europe d. warm climates in northern europe
Answers: 1


You know the right answer?
A3.540-g sample of an unknown metal m is burned in the presence of excess oxygen, producing the oxid...
Questions in other subjects:










Chemistry, 19.03.2020 21:10