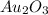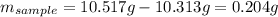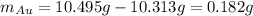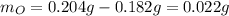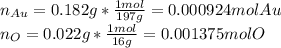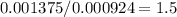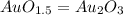Acompound is known to contain only gold and oxygen. a sample of this compound is placed in a clean crucible that has a mass of 10.313 g. the crucible and sample have a mass of 10.517 g. the crucible is heated until the compound decomposes to the elements. the oxygen is lost to the air and the gold remains in the crucible. the mass of the crucible and gold is 10.495 g. what is the empirical formula of this compound?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
Acompound is known to contain only gold and oxygen. a sample of this compound is placed in a clean c...
Questions in other subjects:

Mathematics, 24.07.2019 07:30


Mathematics, 24.07.2019 07:30



Chemistry, 24.07.2019 07:30




Biology, 24.07.2019 07:30