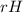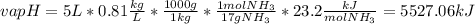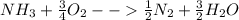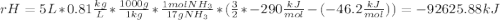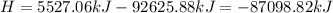Ammonia (nh3) boils at -33∘c; at this temperature it has a density of 0.81 g/cm3. the enthalpy of formation of nh3(g) is -46.2 kj/mol, and the enthalpy of vaporization of nh3(l) is 23.2 kj/mol calculate the enthalpy change when 5 l of liquid nh3 is burned in air to give n2(g) and h2o(g).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Ammonia (nh3) boils at -33∘c; at this temperature it has a density of 0.81 g/cm3. the enthalpy of f...
Questions in other subjects:

Mathematics, 22.02.2020 23:04

Chemistry, 22.02.2020 23:04

History, 22.02.2020 23:04

Physics, 22.02.2020 23:04

Mathematics, 22.02.2020 23:04


Mathematics, 22.02.2020 23:04

Mathematics, 22.02.2020 23:04


Mathematics, 22.02.2020 23:05

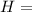 Δ
Δ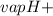 Δ
Δ