
According to the vsepr model, the arrangement of electron pairs around nh3 and ch4 is a. the same, because in each case there are the same number of electron pairs around the central atom b. different or the same, depending on the conditions leading to maximum repulsion c. different, because in each case there are a different number of atoms around the central atom d. the same, because both nitrogen and carbon are both in the second period e. different, because in each case there are a different number of electron pairs around the central atom

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
According to the vsepr model, the arrangement of electron pairs around nh3 and ch4 is a. the same, b...
Questions in other subjects:

English, 26.05.2020 03:01

Mathematics, 26.05.2020 03:01

Chemistry, 26.05.2020 03:01


Advanced Placement (AP), 26.05.2020 03:01

Mathematics, 26.05.2020 03:01


Social Studies, 26.05.2020 03:01


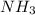 and
and 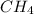 , the number of electron pairs are same but methane has all the four bond pairs where in ammonia, three bond pairs and one lone pair exists. And thus there are repulsion forces possible in between the lone pair and bond pair of electrons thus the arrangement of electron pairs around both the molecules is same or different depending up on the conditions leading to maximum repulsion.
, the number of electron pairs are same but methane has all the four bond pairs where in ammonia, three bond pairs and one lone pair exists. And thus there are repulsion forces possible in between the lone pair and bond pair of electrons thus the arrangement of electron pairs around both the molecules is same or different depending up on the conditions leading to maximum repulsion. 

