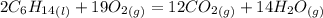Chemistry, 01.11.2019 02:31 twistedgamerhd12
Problem page liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 70. g of hexane is mixed with 81.3 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2


Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
Problem page liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gase...
Questions in other subjects:



Biology, 27.08.2019 09:00




Social Studies, 27.08.2019 09:00



Chemistry, 27.08.2019 09:00