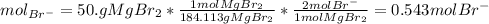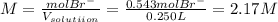Achemist prepares a solution of magnesium bromide by measuring out of into a volumetric flask and filling to the mark with distilled water. calculate the molarity of anions in the chemist's solution. be sure your answer is rounded to the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 00:30, kylee65
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Achemist prepares a solution of magnesium bromide by measuring out of into a volumetric flask and fi...
Questions in other subjects:


History, 10.12.2021 23:40



Mathematics, 10.12.2021 23:40


English, 10.12.2021 23:40

Business, 10.12.2021 23:40


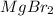 and the volume of the volumetric flask are not given, one can assume arbitrary values and you could modify them whenever you want, thus, let 50.0g of magnesium bromide to be the measured out amount of solute and a 250-mL volumetric flask where the solution is prepared, so the final volume of the solution is 250 mL after the addition of distilled water. In this manner, the bromide anions moles, taking into account there are two bromide moles per magnesium bromide moles, turns out into:
and the volume of the volumetric flask are not given, one can assume arbitrary values and you could modify them whenever you want, thus, let 50.0g of magnesium bromide to be the measured out amount of solute and a 250-mL volumetric flask where the solution is prepared, so the final volume of the solution is 250 mL after the addition of distilled water. In this manner, the bromide anions moles, taking into account there are two bromide moles per magnesium bromide moles, turns out into: