
Chemistry, 01.11.2019 02:31 santileiva123199
Barium can be analyzed by precipitating it as baso4 and determining the mass of the precipitate. when a 0.269 g sample of a barium compound was treated with excess h2so4, 0.0891 g of baso4 formed. what percentage of barium is in the compound?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, wbrandi118
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2


Chemistry, 23.06.2019 10:30, EstherAbuwaah
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh. the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
You know the right answer?
Barium can be analyzed by precipitating it as baso4 and determining the mass of the precipitate. whe...
Questions in other subjects:

English, 03.12.2019 23:31


Mathematics, 03.12.2019 23:31

Biology, 03.12.2019 23:31

English, 03.12.2019 23:31





Health, 03.12.2019 23:31

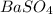 , 1 molecule of
, 1 molecule of 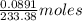 of
of 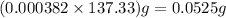
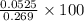 % = 19.5%
% = 19.5%

