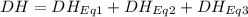At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj eq. 2: 2 o3(g) → 3 o2(g) δh = –284.6 kj eq. 3: 2 o2(g) → 4 o(g) δh = –990.0 kj use the above data to calculate the heat absorbed (kj) when 2.5 moles of no2(g) is formed at room temperature according to the chemical reaction. do not include units. no(g) + o(g) → no2(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 23.06.2019 06:30, Liapis
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2...
Questions in other subjects:




Social Studies, 04.04.2020 12:40





Biology, 04.04.2020 12:41