
Chemistry, 31.10.2019 01:31 CaraRose1887
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both measured at 315 k and 50.0 mmhg), what is the limiting reactant and the theoretical yield of so3? if 187.2 ml of so3 is collected (measured at 315 k and 50.0 mmhg), what is the percent yield for the reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2


Chemistry, 23.06.2019 05:00, sCoTtYbOy5329
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both...
Questions in other subjects:









English, 04.08.2021 04:40

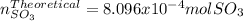
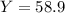 %
%
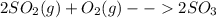
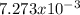 moles of
moles of 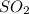 as follows:
as follows: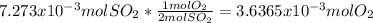
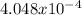 moles are available in comparison with the
moles are available in comparison with the 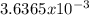 moles that completely would react with
moles that completely would react with 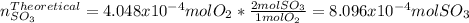
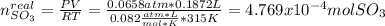
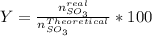 %
%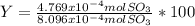 %
%

