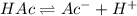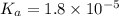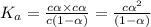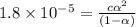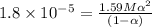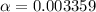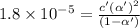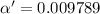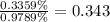Chemistry, 30.10.2019 05:31 skywil8981
Consider that you have two solutions of acetic acid, ka = 1.8x10-5, one solution that is 1.59 m and another solution that is 0.186 m. compare the percent dissociation of acetic acid in these two solutions. what is the ratio of percent dissociation of the 1.59 m solution to the 0.186 m solution? (% dissociation of 1.59 m / % dissociation of 0.186 m) enter your answer numerically to three significant figures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

You know the right answer?
Consider that you have two solutions of acetic acid, ka = 1.8x10-5, one solution that is 1.59 m and...
Questions in other subjects:


Social Studies, 28.11.2019 23:31


Computers and Technology, 28.11.2019 23:31

Mathematics, 28.11.2019 23:31

Mathematics, 28.11.2019 23:31


Social Studies, 28.11.2019 23:31

Mathematics, 28.11.2019 23:31

Mathematics, 28.11.2019 23:31