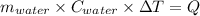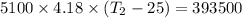Chemistry, 30.10.2019 05:31 mashedpotatoes28
One mole of carbon (12.0 g) in the form of crystalline graphite is burned at 25◦c and 1.000 atm pressure to form co2(g). all of the heat produced is used to heat a 5100 g bath of liquid water, originally at 25◦c. what is the final temperature of the water bath? the heat of formation of co2(g) is −393.5 kj/mol and the specific heat of water is 4.18 j/g/◦c. answer in units of ◦c

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 21:30, rondonalba
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2
You know the right answer?
One mole of carbon (12.0 g) in the form of crystalline graphite is burned at 25◦c and 1.000 atm pres...
Questions in other subjects:





Mathematics, 01.08.2019 22:00



Physics, 01.08.2019 22:00