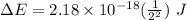Chemistry, 30.10.2019 05:31 htiffany0225
Calculate the energy, in joules, required to ionize a hydrogen atom when its electron is initially in the n =2 energy level. the energy needed to ionize a ground-state hydrogen atom is 2.18 x 10–18 j.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
You know the right answer?
Calculate the energy, in joules, required to ionize a hydrogen atom when its electron is initially i...
Questions in other subjects:


Geography, 15.02.2021 23:30







Mathematics, 15.02.2021 23:30

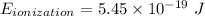
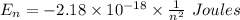
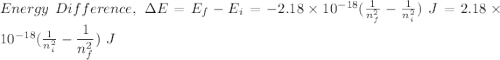
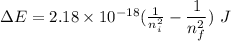
 and
and  (As the hydrogen has to ionize)
(As the hydrogen has to ionize)