
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric acid, that citric acid is a monoprotic acid, and that the density of lemon juice is 1.0 g/ml, then the citric acid concentration calculates to 0.5% by mass. estimate the volume of 0.0100 m naoh required to neutralize a 3.71-g sample of lemon juice. the molar mass of citric acid is 190.12 g/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1


Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric...
Questions in other subjects:









Mathematics, 31.05.2021 15:10

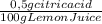 = 0,01855 g of citric acid. In moles:
= 0,01855 g of citric acid. In moles: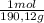 = 9,76x10⁻⁵ moles of citric acid.
= 9,76x10⁻⁵ moles of citric acid.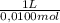 = 9,76x10⁻³L ≡ 9,76 mL
= 9,76x10⁻³L ≡ 9,76 mL


