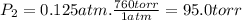Chemistry, 30.10.2019 01:31 alexmoy45p8yd7v
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases. bulb a: 150. ml of co(g) at 190. torr bulb b: 300. ml of ar(g) at 0.500 atm bulb c: 750. ml of kr(g) at 75.994 kpa 1. after both stopcocks are opened and the gases allowed to diffuse throughout, what will be the ultimate total pressure? 2. what is the partial pressure of co(g)?
3. what is the mole fraction of co₂(g)?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, tashanicole
If this equation was completed which statement would it best support
Answers: 1


Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

You know the right answer?
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of t...
Questions in other subjects:


Mathematics, 05.03.2020 05:07


English, 05.03.2020 05:07


Mathematics, 05.03.2020 05:08