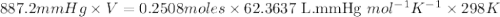Chemistry, 30.10.2019 01:31 scottmichetti
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction include aqueous calcium chloride, liquid water, and gaseous carbon dioxide. calculate the volume of co₂ gas collected over water at 25.0 °c when 25.1 g of calcium carbonate is added to excess hydrochloric acid if the total pressure is 911 mm hg. the vapor pressure of water at 25.0 °c is 23.8 mm hg.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2
You know the right answer?
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction...
Questions in other subjects:








Mathematics, 19.02.2020 21:27


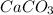 = 100.0869 g/mol
= 100.0869 g/mol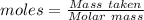
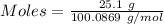
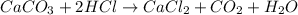
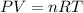
![25^oC=[25+273]K=298K](/tpl/images/0351/9682/df1f6.png)