
Chemistry, 29.10.2019 21:31 Adolfosbaby
A0.5222 g solid sample containing a mixture of lacl3 (molar mass = 245.26 g/mol) and ce(no3)3 (molar mass = 326.13 g/mol) was dissolved in water. the solution was titrated with kio3 , producing the precipitates la(io3)3 and ce(io3)3 . for the complete titration of both la3+ and ce3+ , 42.81 ml of 0.1250 m kio3 was required. calculate the mass fraction of la and ce in the sample.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
A0.5222 g solid sample containing a mixture of lacl3 (molar mass = 245.26 g/mol) and ce(no3)3 (molar...
Questions in other subjects:










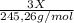 +
+ 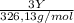 (1)
(1) ×
× = 0,1022g of La
= 0,1022g of La  ×
× = 0,1468g of Ce
= 0,1468g of Ce 

